|
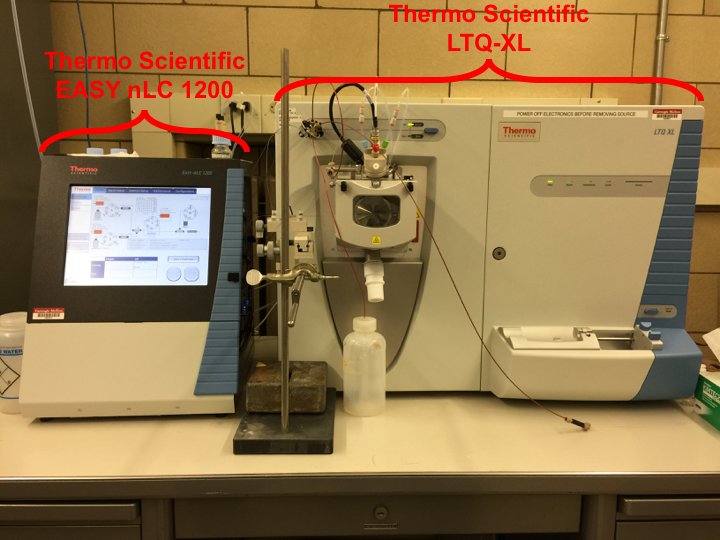
The Thermo Scientific LTQ-XL is a linear ion trap (LIT) mass
spectrometer with electrospray ionization (ESI) and atmospheric pressure
chemical ionization (APCI) capabilities. The LTQ-XL has MSn
capabilities, which is a powerful analytical tool for use in structural
analysis. This mass spectrometer can provide molecular weight and sequence
information for small peptides (MW below 2000 Da) at the femtomole level with
proper analyte introduction. Additionally, liquid seprations prior to MS detection
can be made possible by coupling to the Thermo Scientific EASY-nLC 1200 liquid
chromatograph with precise gradient flow (water/acetonitrile only) from 20 nL/min
to 2 µL/min with pressures up to 1200 bar.
LTQ-XL Linear Iontrap MS USER'S MANUAL in PDF format. Please read for training.
EASY-nLC 1200 USER'S MANUAL in PDF format. Please read prior to nLC-MS inquiries.
System Overview:
· Mass Ranges: 15-200 Th, 50-2000 Th, 200-4000 Th
· Resolution: 0.05 FWHM with Ultra ZoomScan
· Sensitivity: 250 femtogram reserpine in 1:1 isopropanol:water at 400µL/min flow rate for both MS/MS and MSn.
· ZoomScan for isotopic resolution across full-range scan
· Ultra ZoomScan for ultimate resolution
· Turbo Scan for ultra-fast scan to improve signal-to-noise and sampling rate
· Positive/negative ionization modes
· Multiple Ionization Strategies:
· Electrospray Ionization (ESI) -- (Thermo Scientific Ion Max Source)
· Nanoelectrospray Ionization (nESI) -- (Thermo Scientific Nanospray Flex Source)
· Direct Analysis in Real Time (DART) -- (IonSense DART-OS Source)
· Pulsed Q Collision Induced Dissociation (PQD) enables trapping of low mass fragment ions.
· High Resolution Isolation allows for the separation of an isobaric interfering species down to 0.3 Da or for isolation of thermally labile compounds.
· Automatic Gain Control (AGC) used to ensure ideal number of ions stored in linear ion trap each scan.
· MSn for n=1 to 15 for structural analysis
· Advanced Data Dependent Experiments possible (i.e. Triple Play, Neutral Loss, Ion Tree)
· Linear ion trap with radial ejection. Dual conversion dynode detectors used to capture high capacity of the ion signal.
Calibration:
· Automated system calibration performed by CMA
staff only
Sample Preparation:
·
All samples
should be in an ESI-friendly solvent system such as 50/50
Water/Acetonitrile, Water/Methanol, etc.
·
For positive
ion samples, a small amount of acetic or formic acid (0.1% v/v) can be added to
enhance the signal. TFA is not recommended.
·
For direct injection,
prepare at least 100µL of sample. For flow-injection analysis 20µL is
typically sufficient.
·
Recommended
sample concentrations are as follow:
o
Small
organics: 1-10µM
o
Small
peptides: 1-10µM (pmol/µL)
o
Large
proteins: 2-10µM (pmol/µL)
o
Polymers:
10-100µM
NanoLC Overview:
· Pressure range: 0-1200 bar
· Flow range: 20-2000 nl/min (Recommended: 100-1000 nL/min)
· Samples injected via autosampler
· Software integration with MS and instrument control using Xcalibur 4.0
· NanoViper fitting technology enables low dead volume liquid flow path connectivity. Minimizes peak broadening due to mixing.
· nano-ESI ionization source developed in-house utilizes a 10 µm emitter.
Back to Top
|