 |
Research
• Regioregular Poly(3-alkylthiophene)
• End group functionalization
• Side chain functionalization
• Block Copolymer
• Lab Tour

|
 |
 |
Side Chain Functionalization
Regioregular polythiophenes are important building blocks for new electrical applications such as field effect transistors and nanoscale materials. One method that can add complexity to the family of polythiophene is side chain elaboration. A number of functional groups can be added to the 3 position on the thiophene ring. The two major strategies are either to incorporate the desired side chain in the reactive monomer and then polymerized, or to use post polymerizstion reactions add functionality.
One method to add functional groups to the side chain is to utilize post polymerization reactions on poly[3-(6-bromoalkyl)thiophene]1. First 3-(6-bromoalkyl)thiophene) undergoes grignard metathesis reaction2,3 to produce a highly regioregular polymer (HT-Poly(3-bromohexylthiophene). Then, several functional group can be added through a series of reactions. We have successfully substituted several functionalities, such as carboxylic acids, amines, and thiols, as shown in Figure 1. |
.gif)
Figure 1. Post-polymerization Functionalization of Poly(3-(6-bromohexyl) thiophene)
|
Polymer 5 (HT-2,5-Poly(3-octanic acid thiophene) was synthesized by first reacting polymer 1, with excess lithiated 2,4,4-trimethyloxazoline at low temperature to form polymer 2 (HT-2,5-Poly(3-(2(4,4-dimethyloxazolin-2-yl)-heptyl)thiophene), followed by hydrolysis in 3M hydrochloric acid. Synthesis of polymer 6 (HT-2,5-Poly(3-hexylamine thiophene) was accomplished by reacting polymer 1 with sodium azide in DMF at reflux to form the azide derivative, 3 (HT-2,5-Poly(3-hexyl azide thiophene). Subsequently, polymer 3 was reduced with LAH to form the amine. The derivatized polymer 7 (HT-2,5-Poly(3-hexylthiol thiophene), was formed by the reaction of potassium thioacetate with polymer 1 to form the thioester, 4 (HT-2,5-Poly(3-hexylthiolacetate thiophene). Conversion to the thiol was accomplished by reduction with LAH.
The second method to add functionality to the side chains is to first synthesize the monomer with the functional group and then polymerize. This method is used to produce polythiophenes with alkoxy side groups.4 Alkoxy-substituted analogues of PTs exhibit desirable properties, such as reduced band gaps, low oxidation potentials, and a highly stable conducting state.5-7 Here we present the synthesis for a new class of regioregular 3-alkoxy functionalized polythiophenes that exhibit high electrical conductivities and very good stabilities when doped with iodine vapor followed by exposure to ambient conditions.
A series of soluble structurally regioregular alkoxy-substituted PTs (>98% head-to-tail (HT) couplings) of high molecular weights was synthesized through the Grignard Metathesis (GRIM) method2 (Figure 2).
|
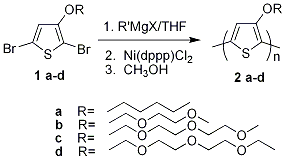
Figure 2. Synthesis of Regioregular Alkoxy-Functionalized Polythiophenes by the Grignard Metathesis (GRIM) Method
|
Dibromoalkoxythiophenes monomers (1a-d) were synthesized first by copper (I) mediated substitution of 3-bromothiophene,8 followed by a modified NBS bromination9 in THF at cryogenic temperatures. The polymerization involved treatment of 1a-d with a Grignard reagent R'MgX (e.g., MeMgBr) that resulted in magnesium bromine exchange, generating a mixture of regiochemical Grignard isomers. Upon addition of Ni(dppp)Cl2, HT-coupled regioregular polymers (2a-d) were produced. |
References
- Zhai, L.; Pilston, R. L.; Zaiger, K. L. ; Stokes, K. K.; McCullough, R. D. Macromolecules, 2003, 36, 61-64.
- Loewe, R.D.; Khersonsky, S. K.; McCullough, R. D. Adv. Mater. 1999, 3, 250.
- Loewe, R. S.; Ewbank, P. C.; Liu, J.; Zhai, L.; McCullough. Macromolecules, 2001, 34, 4324.
- Sheina, E. E.; Khersonsky, S. M.; Jones, E. G.; McCullough, R. D. Chem. Mater. 2005, 17, 317-319
- (a)Jen, K. Y.; Eckardt, H.; Jow, T. R.; Shacklette, L. W.; Elsenbaumer, R. L. J. Chem. Soc., Chem. Commun. 1988, 215. (b) Eckardt, H.; Shacklette, L. W.; Jen, K. Y.; Elsenbaumer, R. L. J. Chem. Phys.1989, 91, 1303. (c) Van Dort, P. C.; Pickett, J. E.; Blohm, M. L. Synth.Met. 1991, 41, 2305.
- (a) Leclerc, M.; Daoust, G. J. Chem. Soc., Chem. Commun. 1990, 273. (b) Daoust, G. J.; Leclerc, M. Macromolecules 1991, 24, 455. (c) Faid, K.; Cloutier, R.; Leclerc, M. Macromolecules 1993, 26, 2501.
- Chen, S. An.; Tsai, C. C. Macromolecules 1993, 26, 2234.
- Keegstra, M. A.; Peters, T. H. A.; Brandsma, L. Tetrahedron 1992, 48, 3633.
- Loewe, R. S.; Ewbank, P. C.; Liu, J.; Zhai, L.; McCullough, R. D. Macromolecules
2001, 34, 4324
|
|
 |
 |

