 |
Research
• Regioregular Poly(3-alkylthiophene) • End group functionalization
• Side chain functionalization
• Block Copolymer
• Lab Tour

|
 |
 |
Regioregular Poly(3-Alkylthiophene)
In the quest for a soluble and processable conducting polythiophene, alkylthiophenes were polymerized. Since 3-alkylthiophene is not a symmetrical molecule, there are three relative orientations available when two thiophene rings are coupled between the 2- and 5-positions. The first of these is 2-5’ or head-to-tail coupling (HT), the second is 2-2’ or head-to-head coupling (HH), and the third is 5-5’ or tail-to-tail coupling (TT). |
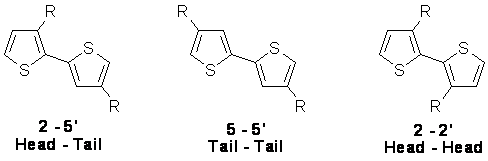
Figure 1. Possible regiochemical couplings of 3-alkylthiophene.
|
Polymers that contain a mixture of the possible couplings are denoted as irregular or non-HT. Irregularly substituted polythiophenes have structures where unfavorable HH couplings cause a sterically driven twist of thiophene rings, resulting in a loss of conjugation. On the other hand, polymers that contain only head-to-tail (HT) couplings are denoted as regioregular. Regioregular poly(3-substituted)thiophene can easily access a low energy planar conformation, leading to highly conjugated polymers. An increase of the torsion angles between thiophene rings leads to greater bandgaps, with consequent destruction of high conductivity and other desirable properties.
|
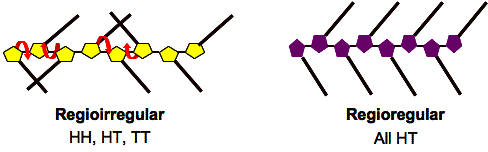
Figure 2. Regioirregular vs. Regioregular poly(3-substitutedthiophene)
|
The first synthesis of regioregular head-to-tail coupled poly(3-alkylthiophenes) (PATs) was reported by McCullough and Lowe2 early in 1992 (Figure 3). Lithiation of 2-bromo-3-alkylthiophenes, using LDA at cryogenic temperatures followed by transmetalation with MgBr2•Et2O generates 2-bromo-5-(bromomagnesio)-3-alkylthiophene. This intermediate is then polymerized with Ni(dppp)Cl2 to give PATs with 98-100% HT-HT couplings.3-7 The second synthetic approach to HT-PAT was subsequently described by Chen and Rieke8 (Figure 3). In this method, Rieke zinc, noted as Zn*, undergoes selective oxidative addition to 2,5-dibromo-3-alkylthiophenes at cryogenic temperatures to afford one regiochemical intermediate. This metalated intermediate undergoes regioselective polymerization to yield the desired HT-PAT |
(1) McCullough Method
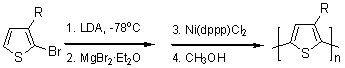
(2) Rieke Method
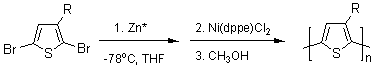
Figure 3. Synthetic methods for regioregular poly(3-alkylthiophene).
|
In 1999, McCullough et. al described another method to produce regioregular H-T coupled poly(3-alkylthiophenes) which can be accomplished at room temperature and at large scale.10,11 The Grignard Metathesis reaction (GRIM) entails the treatment of a 2,5-dibromo-3-alkylthiophene monomer with an equivalant of an alkyl Grignard reagent resulting in a magnesiumbromine exchange reaction. Treatment of this intermediate with a catalytic amount of Ni(dppp)Cl2 affords a egioregular poly(3-alkylthiophene) (Figure 4). The GRIM method is currently being utilized in our lab.
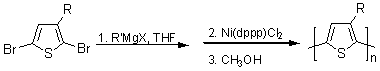
Figure 4. Grignard Metathesis Reaction (GRIM) |
References
- McCullough, R. D. Adv. Mater. 1998, 10, 93.
- McCullough, R. D.; Lowe, R. D. J. Chem. Soc., Chem. Commun. 1992, 70.
- McCullough, R. D.; Lowe, R. D.; Jayaraman, M.;Anderson, D. L. J. Org. Chem. 1993, 58, 904.
- McCullough, R. D.; Lowe, R. D. J. Chem. Soc., Chem. Commun. 1992, 70.
- McCullough, R. D.; Lowe, R. D.; Jayaraman; Ewbank, P. C.; Anderson, D. L.; Tristram-Nagle, S. Synth. Met. 1993, 55, 1198.
- Chen, T.-A.; Rieke, R. D. Synth. Met. 1993, 60, 175.
- Chen, T.-A.; O'Brien, R. A.; Rieke, R. D. Macromolecules. 1993, 26.
- Chen, T.-A.; Rieke, R. D. J. Am. Chem. Soc. 1992, 114, 10087.
- Chen, T.-A.; Wu, X.; Rieke, R. D. J. Am. Chem. Soc. 1995, 117, 233.
- Loewe, R.D.; Khersonsky, S. K.; McCullough, R. D. Adv. Mater. 1999, 3, 250.
- Loewe, R. S.; Ewbank, P. C.; Liu, J.; Zhai, L.; McCullough. Macromolecules, 2001, 34, 4324.
|
| |
|
 |
 |

