Single-Molecule Studies of Heterogeneous Solvation in Ionic Liquids
- Electronic Properties of Molecules Used for Opto-electronic Devices
- Fluorescence Microscopy of Organic Semi-conducting Materials: From Single Molecules to Aggregates
- RNA Splicing at the Single Molecule Level
- Single-Molecule Studies of Heterogeneous Solvation in Ionic Liquids
- Energy Transfer in DNA-based Dye Arrays
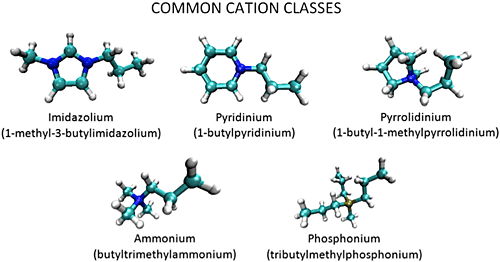
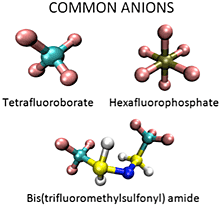 Room temperature ionic liquids (RTILs) are a novel class of green solvents that have a variety of applications ranging from CO2 capture to use as battery electrolytes and in other energy storage devices. They are composed of organic cations and organic or inorganic anions, and have melting points below or near room temperature. RTILs have many desirable properties as solvents for organic reactions including low volatility, non-flammability, good thermal stability, and wide electrochemical windows. However, RTILs exhibit complex solvation dynamics that are still not well understood. For example, spectroscopic studies have shown that dye probes in RTILs exhibit multi-exponential decay dynamics and very broad absorption and emission spectra. This is taken as evidence that molecules within RTILs experience a wide variety of local environments with different solvation properties. Likewise, many simulations on RTILs have observed spatial heterogeneity caused by the aggregation of polar and nonpolar groups. To understand these phenomena at a detailed level, and how they impact the efficacy of ILs in the applications described above, we are studying their structure and solvation dynamics using single-molecule spectroscopy and molecular dynamics simulations through collaboration with the group of Dr. Hyung J. Kim. The single-molecule technique employed in this project is confocal fluorescence lifetime microscopy. Coupled with fluorescence correlation spectroscopy, using both lifetime- and wavelength-selective detection, confocal microscopy gives us the ability to study the local environment around each probe and the dynamics of each individual probe. Combining experiments with simulations, we will achieve a better understand of the interactions between the probes and the ionic liquids and the interactions between the components of ionic liquids themselves.
Room temperature ionic liquids (RTILs) are a novel class of green solvents that have a variety of applications ranging from CO2 capture to use as battery electrolytes and in other energy storage devices. They are composed of organic cations and organic or inorganic anions, and have melting points below or near room temperature. RTILs have many desirable properties as solvents for organic reactions including low volatility, non-flammability, good thermal stability, and wide electrochemical windows. However, RTILs exhibit complex solvation dynamics that are still not well understood. For example, spectroscopic studies have shown that dye probes in RTILs exhibit multi-exponential decay dynamics and very broad absorption and emission spectra. This is taken as evidence that molecules within RTILs experience a wide variety of local environments with different solvation properties. Likewise, many simulations on RTILs have observed spatial heterogeneity caused by the aggregation of polar and nonpolar groups. To understand these phenomena at a detailed level, and how they impact the efficacy of ILs in the applications described above, we are studying their structure and solvation dynamics using single-molecule spectroscopy and molecular dynamics simulations through collaboration with the group of Dr. Hyung J. Kim. The single-molecule technique employed in this project is confocal fluorescence lifetime microscopy. Coupled with fluorescence correlation spectroscopy, using both lifetime- and wavelength-selective detection, confocal microscopy gives us the ability to study the local environment around each probe and the dynamics of each individual probe. Combining experiments with simulations, we will achieve a better understand of the interactions between the probes and the ionic liquids and the interactions between the components of ionic liquids themselves.