Fluorescence Microscopy of Organic Semi-conducting Materials: From Single Molecules to Aggregates
- Electronic Properties of Molecules Used for Opto-electronic Devices
- Fluorescence Microscopy of Organic Semi-conducting Materials: From Single Molecules to Aggregates
- RNA Splicing at the Single Molecule Level
- Single-Molecule Studies of Heterogeneous Solvation in Ionic Liquids
- Energy Transfer in DNA-based Dye Arrays
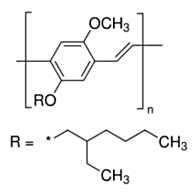
Figure 1a
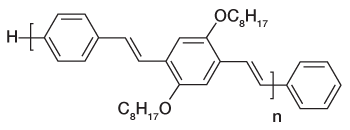
Figure 1b
Recent years has seen a huge growth in the types of organic molecules designed for molecular electronics including organic light emitting diodes (OLEDs) and photovoltaic cells (OPVs). Organic molecules have several advantages over conventional inorganic semi-conductors for these applications including the fact that they can be processed from solution to form flexible films and that elements of organic design can be used to tune properties such as brightness, stability, and the wavelengths of emission to produce devices that emit throughout the visible spectrum. One molecule of this type is the polymer MEH-PPV (see Fig. 1a) and its shorter-chain oligomers (see Fig 1b). When these molecules are formed as thin films, as is necessary to fabricate devices, they typically form aggregates. Aggregates exhibit several desirable properties for device function such as protection from oxidative damage and enhanced charge transport. However, aggregation frequently shifts the wavelength of emission to lower energies and often significantly reduces its intensity. Both of these are undesirable from the standpoint of making organic films for lighting and display applications.
Our group is investigating the relationships between molecular structure (i.e. oligomer length and substitution patterns) and the brightness and photo-stability of molecules in the solid state, both in isolation (i.e. as single molecules) and as aggregates. Molecules that retain a high degree of brightness, even under aggregate conditions, would be good candidates to explore for molecular electronic devices. Through a systematic study of the effect of monomer chain length and aggregate size on photo-stability and brightness, we have identified several that exhibit favorable properties in the aggregate form.
The emission and morphological properties of these aggregates are characterized in bulk solution by a number of techniques including absorption and fluorescence spectroscopies, and fluorescence lifetime measurements. In addition, the properties of single aggregates are probed using single-molecule fluorescence microscopy, spectroscopy, and fluorescence lifetime imaging (Fig. 2). These imaging techniques reveal aggregate-to-aggregate variations in the emission wavelength, brightness, and lifetime. Moreover, we have shown that they are highly sensitive probes of interfacial regions within the aggregates that have distinguishable emission wavelength and lifetime properties (Fig. 3). We are now working to harness this potential to enable high-resolution imaging of thin films in device structures.
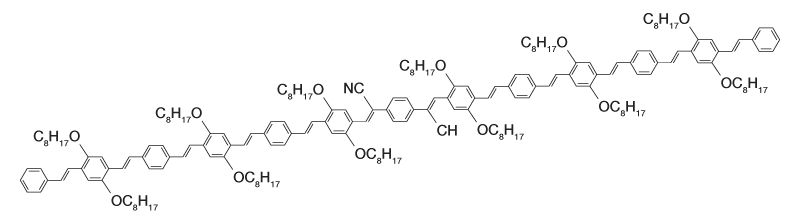
Figure 2a: Molecular structure of oligormeric CN-PPV.
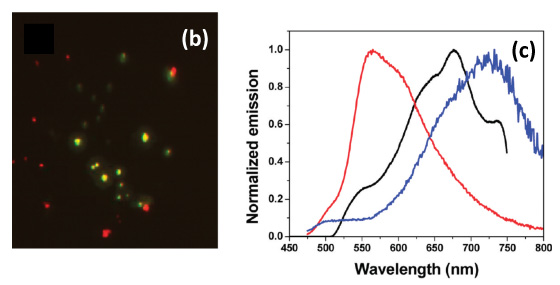
Figure 2: (b) False color microscopy image of red- and green-emitting CN-OPPV13 aggregates obtained by merging images obtained through 700/75 nm and 540/50 nm bandpass filters. (c) Averaged single aggregate emission spectrum (black) compared to spectra of the monomer(red) and aggregates (blue) in MeTHF/MeOH suspension. From J. Phys. Chem. C 2010, 114, 12078
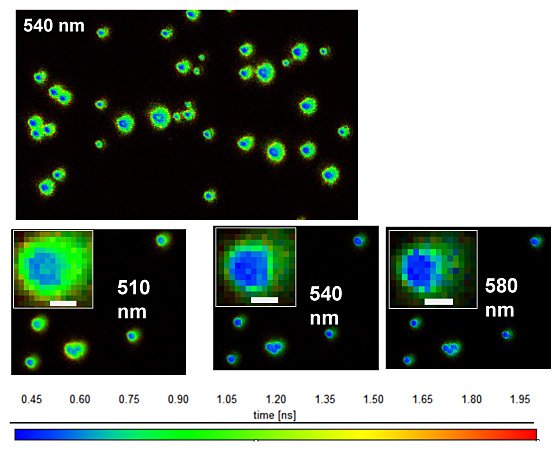
Figure 3: Fluorescence lifetime images of OPPV13 aggregates. Emission wavelengths are indicated (± 10 nm). Images are color coded by average emission lifetime (see scale at bottom of images). From J. Phys. Chem C 2011, 115, 15607