 |
Research
• Regioregular Poly(3-alkylthiophene) • End group functionalization
• Side chain functionalization
• Block Copolymer
• Lab Tour

|
 |
 |
Block Copolymers
Despite their high conductivity and good solubility, regioregular poly(3-alkylthiophenes) do not posses optimal mechanical and processing properties. This issue could be addressed by integrating poly(3-alkylthiophene) in copolymer structures with various polymer blocks that display desirable mechanical properties, leading to a variety ofpolymeric materials with improved properties (e.g. flexible plastics, rigid plastics, elastomers).
The synthesis of diblock copolymers containing head-to-tail-coupled poly(3-alkylthiophenes) (HT-PATs) can be accomplished by executing post polymerization reactions on the end groups of the polymer. The target molecule contains a functional group that intiates atom transfer radical polymerization (ATRP). ATRP is a “living” free-radical polymerization method developed by Matyjaszewski and co-workers to produce a wide variety of conventional polymers1. Two methods of synthesizing diblock copolymers containg regioregular poly(3-alkylthiophene) will be presented.2,3
The first method of synthesis of diblock copolymer described in 2002 is based upon the formation of a HT-PAT with H/Br end groups2 with subsequent end group functionalization reactions4 (Figure 1). Since PATs are chemically stable polymers, the ability to modify the end groups on one side of the polymer is similar to polymer- or bead-supported organic synthesis. Between each reaction, the polymer is isolated, purified by simple precipitation and filtration and characterized by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) and NMR.
|
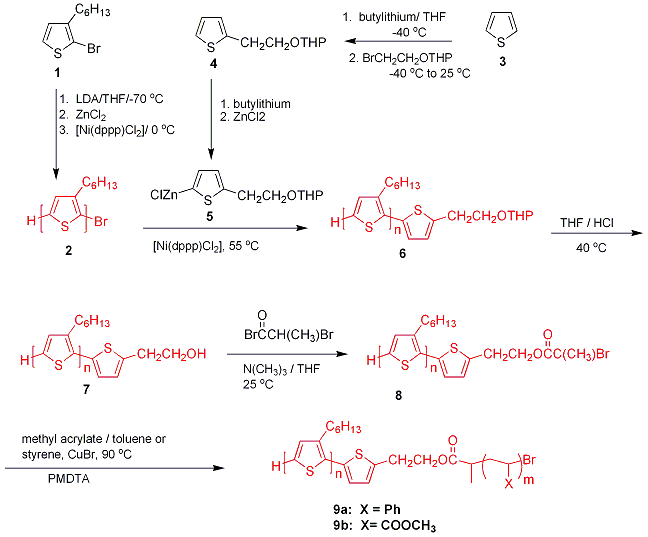
Figure 1. Synthesis of diblock copolymers.
|
The HT-PHT (2) is functionalized by reaction with thiophene derivative (5) and [Ni(dppp)Cl2] (dppp=propane-1,3-diylbis(diphenylphosphane)) to yield polymer 6. Deprotection of 6 leads to HT-PHT with one hydroxyl end group (7). The hydroxy-terminated PHT (7) was further modified by reaction with 2-bromopropionyl bromide to generate an atom transfer radical polymerization (ATRP) macroinitiator (8). The following diblock copolymers has been synthesized: polyhexylthiophene-b- polystyrene (PHT-PS; 9a), and polyhexylthiophene-b- polymethylacrylate (PHT-PMA; 9b) by ATRP using 8 as the initiator, styrene or methyl acrylate as the monomer, and CuBr/N,N,N’,N’,N” -pentamethyldiethylenetriamine (PMDTA) as the catalyst.
Recently our group has developed another method to produce diblock copolymers containing regioregular poly(3-alkylthiophene).3 This method is similar to the above procedure by using post polymerization reactions to create a macroinitiator for ATRP, however the new method involves fewer synthetic steps. A vinyl terminated PHT was used as the diblock copolymer precursor (Figure 2).
The first synthetic step involves the formation of a vinyl terminated PHT via in situ chain end functionalization3, a method developed in our group. The vinyl end group was easily converted to hydroxyethyl end group, that reacts with 2-bromopropionyl bromide to give a bromoester terminated PHT. The latter was used as macroinitiator for ATRP of acrylates.
|
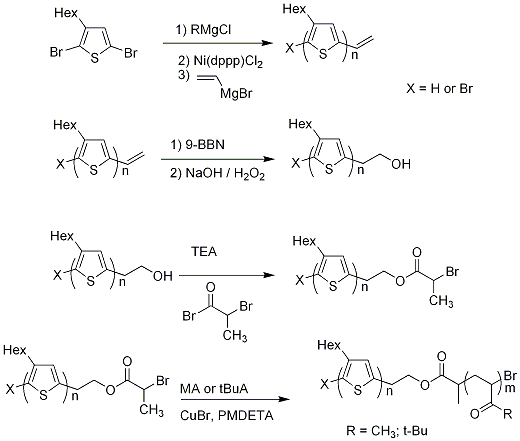
Figure 2. Synthesis of poly(3-hexylthiophene-b-polyacrylates
|
References
- a) Wang, J.-S.; Matyjaszewski, K. J. Am. Chem. Soc. 1997, 119, 5614; b) Patten, T. E.; Xia, J.; Abernathy, T.; Matyjaszewski, K. Science 1996,272, 866; c) Matyjaszewski, K.; Patten, T. E.; Xia, J.J. Am. Chem. Soc.1997, 119, 674; d) Matyjaszewski, K.; Gobelt, B.; Paik, H. –J.; Horwitz, C. P. Macromolecules 2001, 34, 430.
- Liu, J.; Sheina, E.; Kowalewski, T.; McCullough, R. D. Agew. Chem. Int. Ed. 2002, 41,
- Iovu, M. C.; Jeffries-El, M.; Sheina, E. E.; Cooper, J. R.; McCullough, R. D. Polymer, 2005, 46, 8582.
- Liu, J.; McCullough, R. D. Macromolecules 2002, 35, 9882-9889
|
| |
|
 |
 |

