







|
Carnegie Mellon University |
|
I. Physicochemical Aspects of Macrmolecular Self-Assembly in Alzheimer's Disease.
The ultimate objective of this study is to elucidate the physicochemical aspects and molecular mechanisms of pathological self-assembly of biological macromolecules. It is primarily focused on amphipathic peptides and lipoproteins linked to Alzheimer's disease. Special attention is given to the role of interfacial effects and surfaces in aqueous media. This perspective was only recently introduced to the field of physical chemistry of Alzheimer's disease, through our work utilizing in situ atomic force microscopy (AFM). In our studies we are taking advantage of the unique capability of in situ AFM to visualize directly the behavior of biological macromolecules at solid-liquid interfaces, under nearly-physiological conditions.
|
|
Peptides and lipoproteins |
|
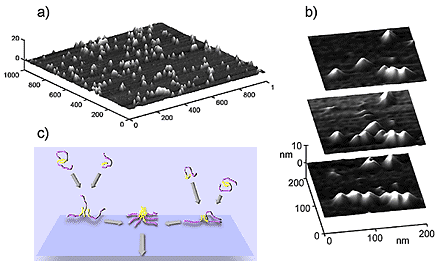
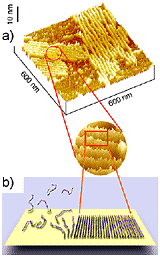
Figure 2. In situ tapping mode AFM of A aggregates assembling in contact with mica: a) three dimensional rendering of the surface of mica covered with globular and protofibrillar aggregates of A; b) time course of the process of assembly of globular aggregates into a linear protofibril (top to bottom, t=512 s; c) schematic illustration of the proposed mechanism of the early stages of formation of pseudomicellar ("mushroom') aggregates of an amphipathic peptide on a hydrophilic substrate. The hydrophilic and hydrophobic portions of peptide chains are colored, respectively, in purple and in yellow (darker and brighter in gray-scale version of the image). |
|
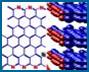
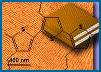
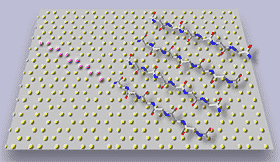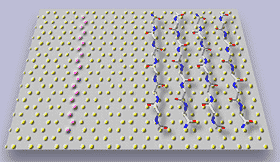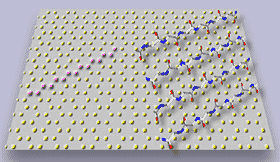
Figure 3. In situ tapping mode AFM of A in contact with graphite: a) two "rafts" consisting of elongated -sheet aggregates of A; b) schematic model illustrating the orientation of peptide chains in the -sheet aggregates; c) The properly scaled model of peptide backbones in an antiparallel sheet arrangement, superimposed on the crystal structure of graphite surface. We propose that the orientation of A aggregates on graphite is driven by a hydrophobic effect and reflects an attempt of the peptide chains to cover the rows of the most densely packed carbon atoms (highlighted in the left portion of a drawing).
T. Kowalewski, D.M. Holtzman PNAS (1999) (You may need a subscription to view this journal paper.) |
|
Room 527, 4400 Fifth Avenue Mellon Institute Pittsburgh, PA 15213 Phone: 412-268-9175 |