Rongchao Jin
Professor
Department of Chemistry
Carnegie Mellon University
4400 Fifth Ave.
Pittsburgh, PA 15213
Office: Mellon Institute 543
Phone: (412) 268-9448
Fax: (412) 268-1061
rongchao@andrew.cmu.edu
Publications
EDUCATION AND PROFESSIONAL EXPERIENCE
- Professor of Chemistry, 2015–present
- Associate Professor of Chemistry, 2012–2015
- Assistant Professor of Chemistry, 2006–2012
- Research Associate, James Franck Institute at the University of Chicago, Illinois, 2003–2006
- Ph.D. Chemistry, Northwestern University, Illinois, 2003
- M.S. Catalysis, Chinese Academy of Sciences/Dalian Institute of Chemical Physics, China, 1998
- B.S. Chemical Physics, University of Science and Technology of China (USTC), China, 1995
SELECTED AWARDS
International Precious Metals Institute (IPMI) Advisor Award (2015), Asian Rising Star Award- Federation of Asian Chemical Societies (2013), Camille Dreyfus Teacher-Scholar Award (2011).
RESEARCH INTERESTS
Our research focuses on fundamental science and engineering questions motivated by the creation of materials at the nanometer scale (1 nm=10-9 m). Our research themes include the synthesis, characterization, and applications of nanoparticles (typically 1-100 nm in size). We develop chemical methods for synthesizing new types of inorganic nanoclusters and nanocrystals, hybrid nano-architectures, and inorganic/polymer nanocomposites. Extensive characterizations of the physical and chemical properties of nanoparticles and self-assembled nanomaterials are carried out with microscopy and spectroscopy techniques, including electron microscopy, X-ray crystallography, steady-state and ultrafast spectroscopies. We develop applications of nanoparticles in areas of catalysis, optics, chemo- and bio-sensing, and energy conversion, etc.
1. Nanocluster and Nanocrystal Chemistry
One of the central themes in nanoscience research is to synthesize high quality nanoparticles with precise control over particle size, shape, structure, and composition. For inorganic nanoparticles (e.g. metal and semiconductor), two regimes are of particular interest, that is, nanoclusters in a size range from subnanometer to ~2 nm and nanocrystals (typically 2-100 nm).
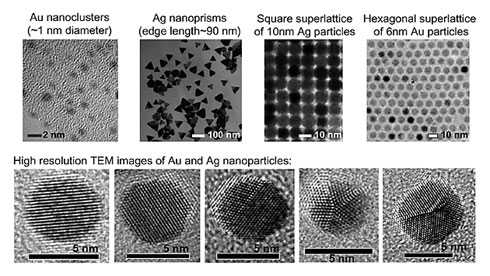
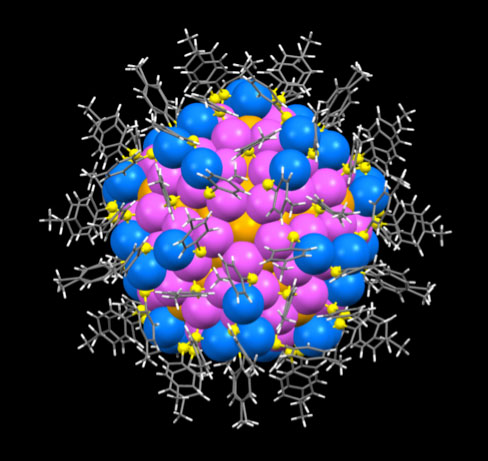
Nanoclusters are comprised of an exact number of atoms, from several to dozens. Recent work has marched toward precise control of giant nanoclusters (>100 atoms). These nanoclusters exhibit fundamentally interesting and important properties (e.g. structural and electronic) different than their larger counterparts—nanocrystals. Molecular chemistry allows the synthesis of organometallic compounds containing few metal atoms, but the metal is often in oxidized states. To make metal nanoclusters Mn (n=10–102) in zero valence state, materials chemistry approach is necessary. Our research goal is to develop new chemical methods to synthesize stable metal nanoclusters with precise control over size, structure, and composition. The unique electronic and surface properties of metal clusters make them very promising in developing a new generation of catalysts that have extraordinary activity and selectivity for a wide range of industrially important chemical processes.
Nanocrystals are typically composed of thousands of atoms and possess a crystalline core (> 2 nm). Controlled synthesis of high quality (e.g. monodisperse and uniform) nanocrystals has been a major research goal in nanoscience. Inorganic nanocrystals and organic nanoparticles are promising in constructing next generation electronic and optoelectronic materials and devices, sensors, and photovoltaic devices. We develop chemical methodologies for synthesizing anisotropic nanocrystals and complex nanocrystal structures as well as nanocrystal/polymer composites for optical and electronic applications. By varying the shape, structural complexity, and chemical composition of the subunits, one can tune the properties of nanomaterials to meet specific needs in practical applications.
2. Linear and Nonlinear Optics of Nanoparticles
Nano-optics, which primarily studies light interaction with nanoparticles, has become a fascinating subfield of nanoscience and nanotechnology. Both nanocluster and nanocrystal-based materials have shown great promise in the development of novel electronic and optoelectronic components or devices, such as optical enhancing materials, sensors, and high-density recording. To realize these goals, it is of fundamental importance to obtain an in-depth understanding of the electronic and optical properties of isolated and assembled nanostructures, such as their charge carrier dynamics and photophysics. We employ steady-state and time-resolved ultrafast spectroscopy in combination with high resolution microscopy to reveal the structure-property relationship of nanomaterials. Understanding such a relationship of nanoparticles and tailoring their properties is a prerequisite to designing novel nanomaterials.
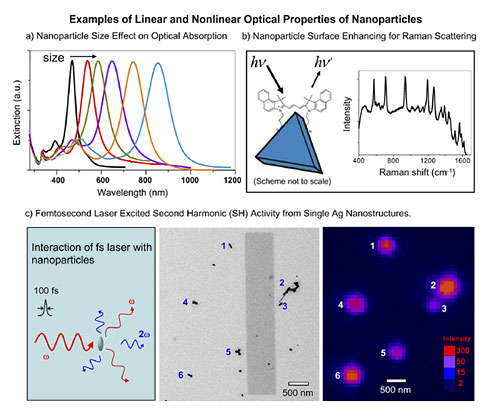
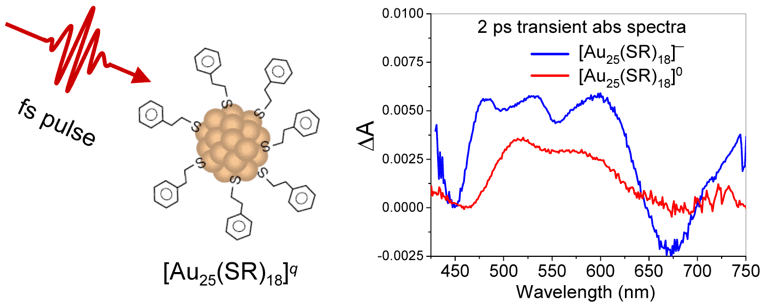
3. NANOCLUSTERS AND NANOPARTICLES FOR CATALYSIS
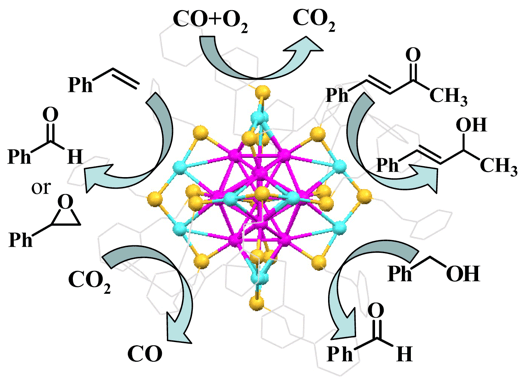 Metal nanoclusters and nanoparticles are of major importance in heterogeneous catalysis. For fundamental studies, the polydispersity of conventional nanocatalysts poses a major issue: the size dependent catalytic activity of nanoparticles is averaged out in polydisperse catalysts, and it is difficult to relate the observed catalytic performance to the structure and intrinsic properties of catalysts. To overcome this obstacle, well-defined catalysts are highly desirable. Recent advances in nanochemistry have led to atomically precise nanoclusters with their total structures characterized by X-ray crystallography. Such nanoclusters hold great promise as a new class of model catalyst for fundamental studies and will provide new opportunities for achieving fundamental understanding of metal nanocatalysis, such as the insight into the size dependence and deep understanding of the molecular activation, catalytically active centers, and catalytic mechanism by correlation with the structures of nanoclusters.
Metal nanoclusters and nanoparticles are of major importance in heterogeneous catalysis. For fundamental studies, the polydispersity of conventional nanocatalysts poses a major issue: the size dependent catalytic activity of nanoparticles is averaged out in polydisperse catalysts, and it is difficult to relate the observed catalytic performance to the structure and intrinsic properties of catalysts. To overcome this obstacle, well-defined catalysts are highly desirable. Recent advances in nanochemistry have led to atomically precise nanoclusters with their total structures characterized by X-ray crystallography. Such nanoclusters hold great promise as a new class of model catalyst for fundamental studies and will provide new opportunities for achieving fundamental understanding of metal nanocatalysis, such as the insight into the size dependence and deep understanding of the molecular activation, catalytically active centers, and catalytic mechanism by correlation with the structures of nanoclusters.