Research Projects
Mechanistic Studies of Non-heme Mononuclear Iron Enzymes
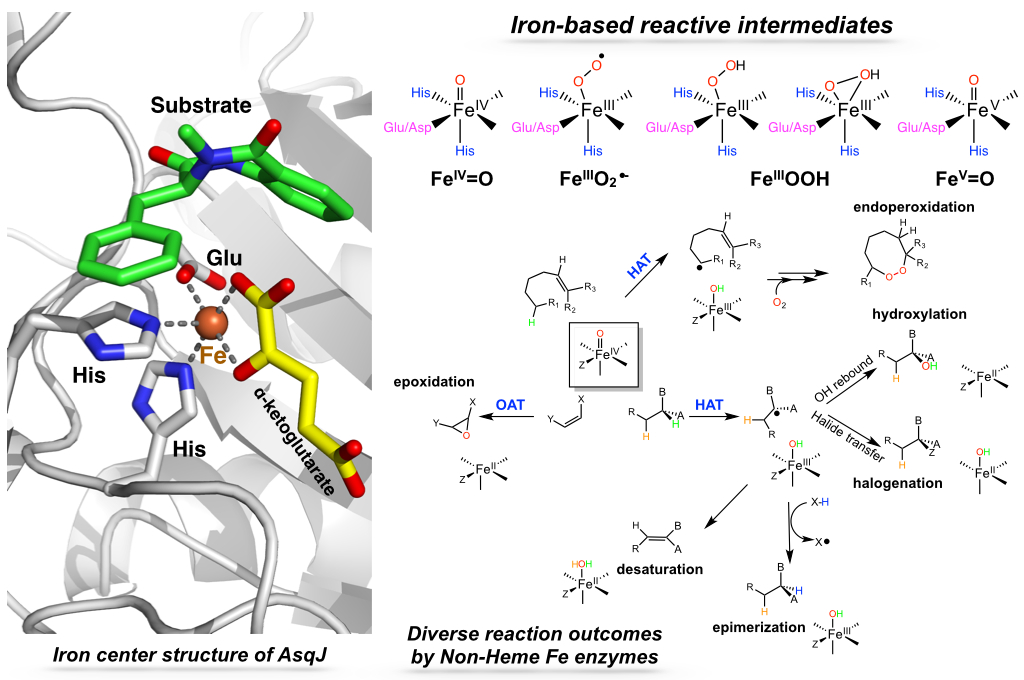
Non-heme mononuclear iron (NHM-Fe) enzymes form a large enzyme family, existing in bacteria, fungi, plants, and animals. By utilizing O2 or H2O2 as the terminal oxidant, they catalyze an exceedingly diverse set of enzymatic reactions, including hydroxylation, halogenation, epimerization, endoperoxidation, desaturation, epoxidation and ring expansion. Many members of this enzyme family are involved in nature product biosynthesis. Of particular interest are enzymes involved in the synthesis of nature products with antibacterial, antimicrobial, and anti-aging activities. Elucidation of their catalytic mechanisms through the detection and characterization of reactive intermediates not only provide key knowledge to understand NHM-Fe enzyme catalysis, but may also offer attractive approaches for plausible biomimetic organic synthetic method development. Mastering these unique biological transformations demonstrated by NHM-Fe enzymes could further lead to the discovery of new bioactive molecules, and thus offering profound implications for human health.
By combining organic synthesis, mechanistic probe design, spectroscopic methods, pre-steady state kinetics, molecular dynamic simulation and density functional theory calculations, we are trying to reveal the molecular level details of the reaction mechanisms of these catalytically versatile NHM-Fe enzymes.
Representative Publications:
Jikun Li, Hsuan-Jen Liao, Yijie Tang, Jhij-Liang Huang, Lide Cha, Te-Sheng Lin, Justin L. Lee, Igor V. Kurnikov, Maria G. Kurnikova, Wei-chen Chang, Nei-Li Chan, Yisong Guo “Epoxidation Catalyzed by the Nonhem Iron(II)- and 2-Oxoglutarate-Dependent Oxygenase, AsqJ: Mechanistic Elucidation of Oxygen Atom Transfer by a Ferryl Intermediate” J. Am. Chem. Soc. 142: 6268–6284, 2020.
Cheng-Ping Yu, Yijie Tang, Lide Cha, Sergey Milikisiyants, Tatyana I. Smirnova, Alex I. Smirnov, Yisong Guo, Wei-chen Chang “Elucidating the Reaction Pathway of Decarboxylation-Assisted Olefination Catalyzed by a Mononuclear Non-Heme Iron Enzyme” J. Am. Chem. Soc. 140: 15190–15193, 2018.
Hsuan-Jen Liao, Jikun Li, Jhih-Liang Huang, Madison Davidson, Igor Kurnikov, Te-sheng Lin, Justin L. Lee, Maria Kurnikova, Yisong Guo, Nei-Li Chan, Wei-chen Chang “Insights into the Desaturation of Cyclopeptin and its C3 Epimer Catalyzed by a non-Heme Iron Enzymes: Structural Characterization and Mechanism Elucidation” Angew. Chem. Int. Ed. 57: 1831–1835, 2018.
Wei-chen Chang, Jikun Li, Justin L. Lee, Andrea A. Cronican, Yisong Guo “Mechanistic Investigation of a Non-Heme Iron Enzyme Catalyzed Epoxidation in (-)-4’-Methoxycyclopenin Biosynthesis” J. Am. Chem. Soc.138: 10390–10393, 2016.
Wei-chen Chang, Yisong Guo, Chen Wang, Susan E. Butch, Amy C. Rosenzweig, Amie K. Boal, Carsten Krebs, J. Martin Bollinger, Jr. “Mechanism of the C5 Stereo-inversion Reaction in the Biosynthesis of Carbapenem Antibiotics” Science, 343: 1040–1144, 2014.
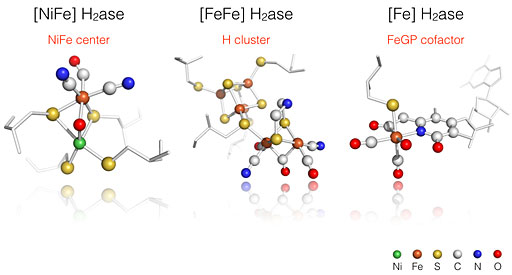
H2 Activation and Production by Hydrogenases
H2 has been considered as the fuel of future due to its high energy density (140 MJ/Kg) and zero carbon emission (water is the only end product). However, the production and utilization of H2 as an energy carrier is still at the initial stage, many scientific and technological developments need to be carried out. In nature, H2 is used as a source of electrons and produced as electron acceptor in various organisms through a class of metalloproteins, called hydrogenases (H2ases). The extremely high turn over rate of some H2ases (up to 103 – 104 H2 molecules per second!) has drawn much attention from chemists, biochemists, and bioengineers to understand the reaction mechanism of this class of enzymes. This knowledge will provide guidelines to design and synthesize high performance low cost H2 evolving catalysts that can operate under aqueous solution and neutral pH for large-scale industrial applications.
Using steady state, pre-steady state kinetics, spectroscopic tools and density functional theory (DFT) calculations, we are trying to capture and characterize catalytic intermediates down to fundamental chemical steps.
Representative Publications:
David W. Mulder, Yisong Guo, Michael W. Ratzloff, Paul W. King “Identification of a Catalytic Iron-Hydride at the H-cluster of [FeFe]-Hydrogenase” J. Am. Chem. Soc.139: 83–86, 2017.
Yisong Guo, Hongxin Wang, Yuming Xiao, Sonja Vogt, Rudolf K. Thauer, Seigo Shima, Phillip I. Volkers, Thomas B. Rauchfuss, Vladimir Pelmentschikov, David A. Case, Ercan E. Alp, Wolfgang Sturhahn, Yoshitaka Yoda, and Stephen P. Cramer ‘Characterization of the Fe Site in Methanothermobacter marburgensis Hydrogenase (mHmd) via Nuclear Resonance Vibrational Spectroscopy (NRVS)’ Inorg. Chem. 47: 3969–3977, 2008.
Iron-Sulfur Cluster Chemistry
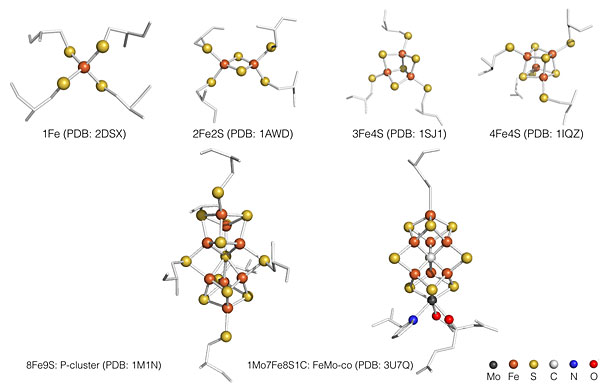
Iron-sulfur clusters are one of the most ancient, versatile and multi-functional prosthetic groups found in biology. They are commonly involved in essential biological processes in living cells, such as electron transport, catalysis, and regulatory sensing (See figure below for commonly encountered Iron-sulfur clusters in biology). The deficiency of iron-sulfur clusters is also believed to link to many human diseases, such as Friedreich’s ataxia. Their unique structures give rise to very interesting electronic behaviors, which have been described by exchange, double exchange, and vibronic coupling between different irons in the same cluster. These behaviors, in turn, determine the detailed structures of iron sulfur clusters as well as their modes of action.
We are interested in identifying new functions and revealing structural basis to support these new functions. We are also interested in bio-assembly of iron-sulfur clusters in nature. FeMo-co and P-cluster from nitrogenase enzyme are two of the most complicated iron-sulfur clusters found in biology. There are more than dozens of gene products responsible for the maturation of these unique clusters in vivo. Using Mössbauer and EPR, we are trying to elucidate electronic structures of intermediate clusters along the bio-assembly pathway, and further deduce their geometric structures.
Representative Publications:
Yisong Guo, Carlos Echavarri-Erasun, Marie Demuez, Emilio Jiménez-Vicente, Emile L. Bominaar, Luis M. Rubio “The Nitrogenase FeMo-cofactor Precursor Formed by NifB Protein: A Diamagnetic Eight Iron Atoms” Angew. Chem. Int. Ed. 41: 12764–12767, 2016.
Aubrey D. Scott, Vladimir Pelmenschikov, Yisong Guo, Lifen Yan, Hongxin Wang, Simon J. George, Christie H. Dapper, William E. Newton, Yoshitaka Yoda, Yoshihito Tanaka, Stephen P. Cramer* “Structural Characterization of CO-Inhibited Mo-Nitrogenase by Combined Application of Nuclear Resonance Vibrational Spectroscopy, Extended X-ray Absorption Fine Structure, and Density Functional Theory: New Insights into the Effects of CO Binding and the Role of the Interstitial Atom” J. Am. Chem. Soc. 136: 15942–15954, 2014.
Synchrotron Mössbauer Spectroscopy and Imaging
Mössbauer Spectroscopy is one of the most frequently utilized tools in the study of iron centers of metalloenzymes. It detects all iron in a given sample, and determines their oxidation states, spin states, and precise quantification of iron in different local environments. The development of third generation synchrotron radiation (SR) sources and X-ray optics brings synchrotron Mössbauer spectroscopy (SMS) into reality. The high brightness (billion times more intense than lab-based radiation sources) and small beam size (sub micrometers to nanometers) of SR give Mössbauer a brand new future.
Our goal is to develop SMS into an in vivo imaging tool. The advantage of SMS imaging is that it not only can probe iron distributions in organelles and whole cells, it can also measure Mössbauer spectra at any spatial point and reveal nature of iron species located there.
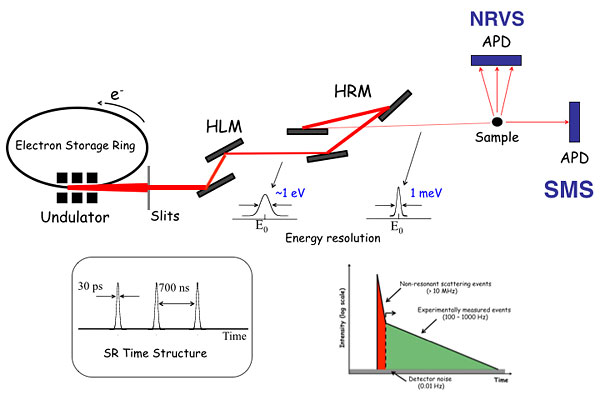
Experimental setup of synchrotron Mössbauer spectroscopy (SMS) and nuclear resonance vibrational spectroscopy (NRVS)
Representative Publications:
Andrew C. Weitz, Ethan A. Hill, Victoria F. Oswald, Emile L. Bominaar, Andy S. Borovik, Michael P. Hendrich, Yisong Guo “Probing Hydrogen Bonding Interactions to Iron-Oxido/Hydroxido Units via 57Fe Nuclear Resonance Vibrational Spectroscopy” Angew. Chem. Int. Ed. 57: 16010–16014, 2018.
Yisong Guo, Yoshitaka Yoda, Xiaowei Zhang, Yuming Xiao, Stephen P. Cramer “Synchrotron Radiation Based Nuclear Resonant Scattering: Applications to Bioinorganic Chemistry” Book Chapter in Mössbauer Spectroscopy: Applications in Chemistry, Biology, Nanotechnology, Industry, and Environment, Wiley, 2013.