ResearchSupramolecular Assemblies Containing Transition Metal IonsProgress in supramolecular chemistry is driven by the human quest for sophisticated, high-symmetry structures and by the possible applications these structures have in fields like materials science, chemical technology, and medicine. Structures containing transition metal ions are of particular interest because of special magnetic and electric properties conferred upon the supramolecular assemblies by the presence of multiple metal paramagnetic centers. Metal ions have been used for "programmed" reading of the information contained in ligands with multiple coordination sites, which is essential for directing the organization of elaborate structures in self-assembly processes. One of the most interesting structures pursued in supramolecular chemistry is that of helix. DNA is the classical example of biological helix formed by using a network of hydrogen bonds and base stacking interactions.
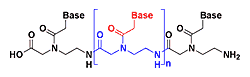
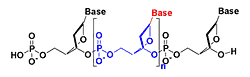
The goal of our research is to create novel nanosize structures that contain transition metal ions and have potential nanotechnology applications. One strategy for creating such structures is based on the use of the molecular recognition properties of transition metal ions and peptide nucleic acids (PNA). PNA is a synthetic analogue of DNA that has a pseudo-peptide backbone (blue in Figure 2a) instead of the sugar phosphate backbone backbone of DNA (blue in Fig. 2b)
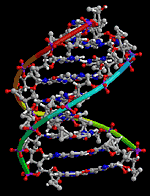
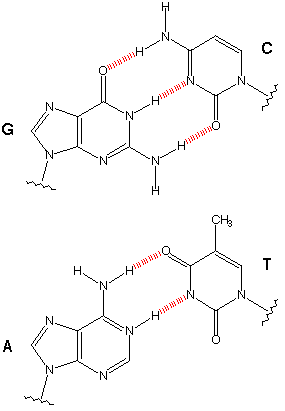
In PNA, nucleobases are linked to the backbone by amide bonds and PNA oligomers form a helical double helix by Watson-Crick base pairing (Figure 3).
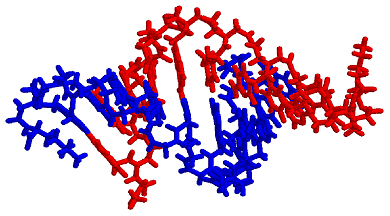
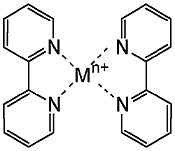
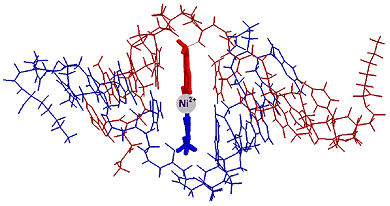
Substitution of bipyridine for a nucleobase leads to modified peptide nucleic acid (PNA) single strands that are bridged in the presence of Ni2+ into a duplex containing a combination of hydrogen (Fig. 4a) and coordinative bonds (Fig. 4b).
CD experiments demonstrate that the duplex adopts a structure similar to that of an unmodified 10-bp PNA duplex and UV melting experiments show a very sensitive dependence of the duplex stability on the substitution of a nucleobase pair with a pair of ligands or a metal-ligand alternative base pair.2 The spatial arrangement and dimensions of the nanostructures is determined by the coordination properties of transition metal ions and by the structure of PNA, a synthetic analogue of DNA. Metal ion incorporation in PNA duplexes is achieved by substituting ligands for natural nucleobases. This procedure allows the precise control of the number and position of metal ions in the PNA duplexes. Formation of duplexes or higher complexity structures is driven by hydrogen bonds between the natural nucleobases and coordinative bonds between the ligands incorporated in PNA strands and the metal ions bridging these strands. References
|
Research Projects |
|||||||||||||||||||||||||
|
||||||||||||||||||||||||||