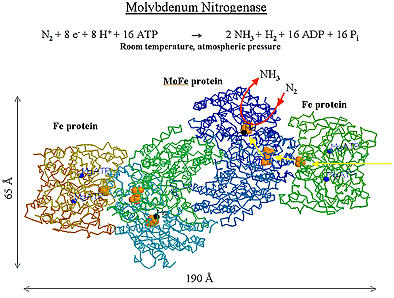
ResearchMagneto-electronics of Transition-metal ClustersOne of the most important functions of metal cofactors in biological systems is electron transfer. This is a function that metal complexes also exert in homogeneous catalysis. The relationship between the electronic structure of metal centers and their electron-transfer properties is relevant for the specificity of redox partners and the reaction pathways in multi-center enzymes. Figure 1 represents an example of the most elaborate biological catalysts, the MoFe nitrogenase. This enzyme is part of a complex machinery which ensures the electron transfer required for nitrogen conversion to ammonia. The
The quest for understanding biological electron transfer lead to numerous experimental and theoretical studies, which addressed primarily the contributions of mononuclear metal sites to- and the influence of protein on- electron transfer. In contrast, aspects of electron transfer specific for polynuclear metal cofactors have been much less studied. Polynuclear clusters are redox centers in iron–sulfur proteins, iron–oxo proteins, cytochrome c oxidase, and the Mn water-oxidation catalyst of photosystem II. In most cases, clusters contain paramagnetic metal sites and exchange coupling of the unpaired electrons at these sites gives rise to a property specific to clusters, namely the presence of a relatively closely-spaced number of spin states. Moreover, in one-electron transfer processes, mixed-valence states of the clusters are attained and in these states spin-dependent intramolecular electron transfer occurs. To present, the influence of exchange interactions on the thermodynamics and kinetics of electron-transfer have been only the subject of theoretical studies. Investigation of the effect of exchange interactions in biological clusters on any measured property is difficult because it requires deconvolution of the cluster contributions from contributions of protein and of adventitiously bound metal ions. In this research project we design and synthesize polynuclear coordination complexes to be used in the rational investigation of the effect of spin state on the kinetics of electron transfer. The research involves the use of polynuclear metal clusters which in the same topology exhibit significantly different spin ladders and which can be subject to electron-transfer kinetics investigations. References
|
Research Projects |
||||||||||
|
|||||||||||