|
Iron Sulfur Clusters
We
have been fortunate to be involved in characterizing novel structures such
as the nitrogenase M and P-centers,1 the coupled heme-[4Fe-4S]
chromophore of E. coli. sulfite
reductase,2 [3Fe-4S] clusters,3
the clusters of carbon monoxide dehydrogenase,4 the key FeIVFeIV intermediate (compound Q) in the catalytic cycle of
methane monooxygenase,5 the H-cluster of [Fe]-hydrogenases,6
and the oxygen sensor of E. coli.7 For well established
structures, we have been interested in attaining new oxidation states, e.g.
the all-ferrous [4Fe-4S] cluster.8 In general terms, our
interests are described in Ref 9. We continue to study a variety of iron-sulfur
proteins.
|
|
Our idea of the nitrogenase P-cluster shortly
before crystallographic refinement by Rees et al. (1992)
|
(1)
Surerus, K. K.; Hendrich,
M. P.; Christie, P.; Rottgardt, D.; Orme-Johnson, W. H.; Münck,
E. J. Am. Chem. Soc. 1992, 114, 8579-8590.
(2) Bominaar, E. L.; Hu, Z.; Münck, E; Girerd, J.-J.; Borshch, S. J.
Am. Chem. Soc. 1995, 117, 6976-89.
(3) Emptage, M. H.;
Kent, T. A.; Huynh, B. H.; Rawlings, J.; Orme-Johnson,
W.H.; Münck, E. J. Biol. Chem. 1980,
255, 1793-1796.
(4) Xia, J.; Hu,
Z.; Popescu, C. V.; Lindahl,
P. A.; Münck, E. J. Am. Chem. Soc . 1997,
119, 8301-12.
(5) Shu, L.; Nesheim, J. C.; Kauffmann, K.; Münck,
E.; Lipscomb, J. D.; Que Jr., L. Science
1997, 275, 515-518.
(6) Popescu, C. V.;
Münck, E. J. Am. Chem. Soc.
1999, 121, 7877-7884.
(7) Popescu, C.;
Bates, D. M.; Beinert, H.; Münck,
E.; Kiley, P. J. Proc. Natl. Acad. Sci. USA 1998,
95, 13431-13435.
(8) Yoo, S. J.; Angove
H. C.; Burgess, B.K.; Hendrich, M.P.; Münck, E. J. Am. Chem. Soc. 1999,
121, 2534-2545.
(9) Beinert, H.; Holm,
R.H.; Münck, E. "Iron-Sulfur Clusters:
Nature’s Modular Multipurpose Structures" Science 1997,
277, 653-659.
(10) Chakrabati, M.; Deng, L.; Holm,
R. H.; Munck, E.; Bominaar,
E. L. Inorg. Chem. 2009,
48, 2735-2727 (Cover page Inorg.
Chem. Apr. 6, 2009).
|
|
High-Valent
complexes of Biological Relevance
Together with the
group of Lawrence Que, Jr., at the University of Minnesota, we are
studying the electronic structures of high-valent
iron complexes relevant to oxygen activation. Our joint projects have
produced a larger number of interesting compounds. Among these is the first
nonheme FeIV-oxo
complex,1 [FeIV(O)(TMC)(NCMe)]2+.
This complex has electronic spin S = 1. For nonheme
proteins the combination of carboxylate, histidine, H2O, OH- ligands generally produces high-spin (S = 2) FeIV sites, an environment not easily
produced in a synthetic complex.
Collaborating
with the groups of T. J. Collins here at CMU and L. Que,
Jr. we reported in 2008 a comprehensive spectroscopic study of the first FeV=O complex, using the Collins TAML ligand (figure 2).2 In
2012 we followed with the second FeV=O
complex.3 This complex was generated by reacting [FeIV(O)(TMC)(NCMe)]2+
at -44 °C with tert-butyl hydroperoxide
in the presence of a strong base. The new species exhibits in the glass-forming
3:1 butyronitrile/MeCN
solvent an S = 1/2 EPR signal with very narrow lines (4 gauss). The
high-resolution allowed mapping of 14N, 57Fe and
17O hyperfine tensors by EPR. It required detailed DFT analysis
(with choice of a suitable functional) to interpret the Mossbauer, EPR and
resonance Raman data in a consistent way. The S = 1/2 species turned out to
be an iron(V) complex having axial oxo and acetylimido ligands, namely [FeV(O)(TMC)(NC(O)CH3)]+.
We are currently pursuing other systems for which FeV=O
species are indicated, including complexes implicated in water oxidation.
We
have recently studied a group of high-valent diiron complexes based on the TPA ligand
(traded by insiders as the Castro Brothers). The FeIVFeIV
complex has two local S = 1 sites which are ferromagnetically
coupled to yield an S = 2 system state.4 One site, Feb,
has a terminal oxo group; Fea
has a hydroxo ligand.
(figure 3) Given that the Fe-O-Fe angle is 130°, the observation of
ferromagnetic coupling was puzzling, but could be explained quite well
after realizing that the two sites have different ligand
fields that produce a crucial pair of orthogonal magnetic orbitals. Reduction by one electron renders Fea site high-spin FeIII.
Concomitantly Feb undergoes a transition to high-spin (Sb=2)
FeIV=O, a transition that is driven by
superexchange interactions between Fea and Feb (By going
high-spin, the FeIV=O site enables
efficient antiferromagnetic pathways between the
two Fe).5 For the same TPA ligand our
collaborators have produced dinuclear complexes
with open and closed cores, such as O=FeIV-O-FeIV-OH and FeIV(µ-O)2FeIV.
Core opening increases H-bond cleaving reactivity roughly 1000-fold; the
spin transition at the oxo site yields an
additional 1000-fold increase (see Fig. 4 of ref 6).
|
|

[FeIV(O)(TMC)(NCMe)]2+
|
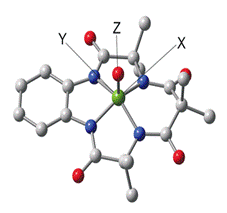
(TAML)FeV=O
|
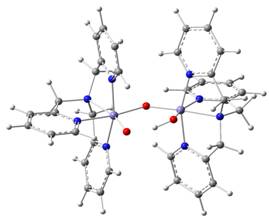
(TPA)(O)FeIV-O-FeIV(OH)(TPA)
|
|
(1) Rohde, J.-U.; In, J.-H.; Lim, M. H.; Brennessel, W. W.; Bukowsky,
M. R.; Stubna, A.; Münck,
E.; Nam, W.; Que Jr., L. Science 2003,
299, 1037-1039.
(2) Tiago de Oliveira,
F.; Chanda, A.; Banerjee,
D.; Mondal, D.; Bominaar,
E.; Münck, E.; Collins, T. C. Science 2006, 315, 835-838. (PMID: 17185561)
(3) Van Heuvelen, K. M.; Fiedler, A. T.; Shan, X.; De Hont, R. F.;
Meier, K. K.; Bominaar, E. L.; Münck, E.; Que, Jr., L. Proc. Natl. Acad. Sci
.2012, 109, 1193-38.
(4) Martinho,
M.; Xue, G.; Fiedler, A. T.; Que,
Jr., L.; Bominaar, E. L.; Münck,
E. J. Am. Chem. Soc. 2009,
131, 5823-5830.
(5) De Hont, R. F.; Xue, G.; Hendrich, M. P.; Que, L. Jr.;
Bominaar, E. L.; Munck,
E. Inorg. Chem. 2010, 49, 8310-8322.
(6) Xue,
G.; De Hont, R. F.; Münck,
E.; Que, L. Jr. Nature Chem. 2010,
2, 400-405.
|
|
Dioxygenases and Monooxygenases
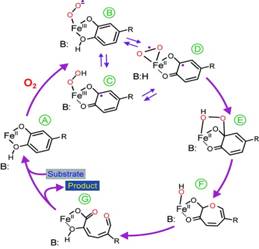 Together with
the group of J. D. Lipscomb at the University of Minnesota we are studying
various oxygen activating enzymes, among them the Fe2+ homoprotocatechuate 2,3 dioxygenase
(2,3 HPCD) which cleaves the aromatic ring of homoprotocatechate
(HPCA) adjacent to the vicinal hydroxyl groups. This enzyme represents a
large group of dioxygenases involved in bacterial
degradation pathways of natural and man-made compounds. In the native state
of 2,3 HPCD the Together with
the group of J. D. Lipscomb at the University of Minnesota we are studying
various oxygen activating enzymes, among them the Fe2+ homoprotocatechuate 2,3 dioxygenase
(2,3 HPCD) which cleaves the aromatic ring of homoprotocatechate
(HPCA) adjacent to the vicinal hydroxyl groups. This enzyme represents a
large group of dioxygenases involved in bacterial
degradation pathways of natural and man-made compounds. In the native state
of 2,3 HPCD the 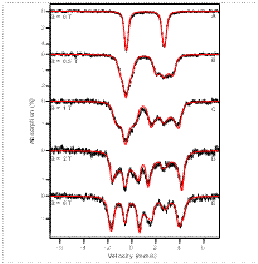 Fe2+
is coordinated by a 2-His-1-Glu facial triad; the three remaining
coordination sites are occupied by water molecules which are displaced upon
(bidentate) substrate and O2 binding.
The figure summarizes current ideas of the mechanism. After oxygen binds,
an electron is transferred through the iron to the oxygen, giving both
substrates radical character (SQ· -FeII-O2·-
in step D ). Recombination of the
radicals would yield an alkylperoxo intermediate,
step E. A subsequent Criegee-type rearrangement would result in O-O bond
cleaving to yield a lactone intermediate, with
the second oxygen retained on the iron. Hydrolysis of the lactone by this oxygen would then yield the product. Fe2+
is coordinated by a 2-His-1-Glu facial triad; the three remaining
coordination sites are occupied by water molecules which are displaced upon
(bidentate) substrate and O2 binding.
The figure summarizes current ideas of the mechanism. After oxygen binds,
an electron is transferred through the iron to the oxygen, giving both
substrates radical character (SQ· -FeII-O2·-
in step D ). Recombination of the
radicals would yield an alkylperoxo intermediate,
step E. A subsequent Criegee-type rearrangement would result in O-O bond
cleaving to yield a lactone intermediate, with
the second oxygen retained on the iron. Hydrolysis of the lactone by this oxygen would then yield the product.
For the native enzyme and three mutants
we have followed the catalytic reaction with rapid freeze-quench Mössbauer and EPR spectroscopy and characterized a
variety of intermediates, among them an Fe3+-superoxo species and a semiquinone-Fe3+-hydroperoxide complex (1-3). For native HPCD
and its mutants, E. Kovaleva and J. D. Lipscomb
have obtained >
50 high-resolution X-structures as well as a wealth of kinetic data. For a
variety of states we have obtained well resolved Mössbauer
spectra which, in conjunction with quantum chemical calculations, can be
used to gain insight into the electronic structures of crucial
intermediates. This will allow us to relate electronic and X-ray structure
data along the reaction pathways and thus obtain crucial information about
reactivity. The figure shows Mössbauer spectra of
an E-S complex; red lines: spectral simulations).
We are continuing ongoing studies aimed
at characterizing
intermediates of the reaction cycle of methane monooxygenase, such as species Q, Q' and P*.
(1)
Mbughuni, M. M., Chakrabarti, M., Hayden, J. A., Bominaar,
E. L., Hendrich, M. P., Münck,
E., and Lipscomb, J. D. (2010) Trapping and spectroscopic characterization
of an FeIII-superoxo intermediate from
a nonheme mononuclear iron-containing enzyme. Proc.
Natl. Acad. Sci. U. S. A. 107, 16788-16793.
(2)
Mbughuni, M. M., Chakrabarti, M., Hayden, J. A., Meier, K. K., Dalluge, J. J., Hendrich, M.
P., Münck, E., and Lipscomb, J. D. (2011)
Oxy-intermediates of homoprotocatechuate
2,3-dioxygenase: Facile electron transfer between substrates. Biochemistry
50, 10262-10274.
(3)
Mbughuni, M. M.; Meier, K. K.; Münck, E.; Lipscomb, J. D. "Substrate-Mediated Oxygen Activation by Homoprotocatechuate 2,3-Dioxygenase:
Intermediates Formed by a Tyrosine 257 Variant" Biochemistry 2012,51, 8743-54.
Electronic Structure Analysis
The
spectroscopic studies of our group have often been complemented by DFT
calculations. These computations provide theoretical estimates of
experimentally determined spin-Hamiltonian parameters, such as zero-field splittings, exchange-coupling constants, 57Fe
isomer shifts, quadrupole splittings,
and magnetic hyperfine coupling constants, and give detailed insights into
the dependency of these parameters on molecular geometry and electronic
structure. These studies have clarified the origin of the unquenched
orbital momentum in the diketiminate complexes of
iron,1,2 the intrinsic mechanism for the distortion of the
Fe(SR)4 center in rubredoxins,3 the influence of
excited spin triplet states on the zero-field splitting in reduced form of
these centers,4 and the oxidation state of the cofactor in
nitrogenase.5
(1) Andres, H.; Bominaar,
E.L.; Smith, J.M.; Eckert, N.A.; Holland, P.L.; Münck,
E. J. Am. Chem. Soc. 2002, 124, 3012-3025.
(2) Stoian, S.A.; Yu, Y.; Smith,
J.M.; Holland, P.L.; Bominaar, E.L.; Münck, E. Inorg. Chem. 2005,
44, 4915-4922.
(3) Vrajmasu, V.V.; Münck, E.; Bominaar, E.L. Inorg. Chem. 2004,
43, 4862-4866; ibid. 4867-4879.
(4) Vrajmasu, V.V.; Bominaar, E.L.; Meyer, J.; Münck,
E. Inorg. Chem. 2002,
41, 6358-6371.
(5) Vrajmasu, V.V.; Münck, E.; Bominaar, E.L. Inorg. Chem. 2003,
42, 5974-5988.
|
|





 Together with
the group of J. D. Lipscomb at the University of Minnesota we are studying
various oxygen activating enzymes, among them the Fe2+
Together with
the group of J. D. Lipscomb at the University of Minnesota we are studying
various oxygen activating enzymes, among them the Fe2+  Fe2+
is coordinated by a 2-His-1-Glu facial triad; the three remaining
coordination sites are occupied by water molecules which are displaced upon
(
Fe2+
is coordinated by a 2-His-1-Glu facial triad; the three remaining
coordination sites are occupied by water molecules which are displaced upon
(